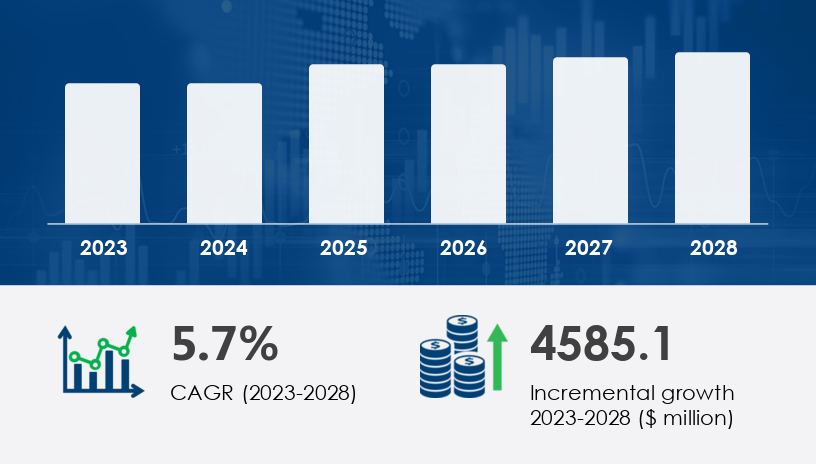
The global rare hematology market is poised for significant growth, with projections indicating an increase of USD 4.59 billion at a CAGR of 5.7% from 2023 to 2028. This expansion is driven by advancements in gene therapies, regulatory approvals, and a shift towards patient-centric treatment paradigms. However, the market faces challenges, notably the high costs associated with these innovative therapies.
Rare hematology encompasses a range of uncommon blood disorders, including hemophilia, sickle cell disease, thalassemia, myelodysplastic syndromes, and various plasma disorders. The increasing prevalence of these conditions necessitates the development of specialized treatments and diagnostic tools.
For more details about the industry, get the PDF sample report for free
The rare hematology market is driven by the growing prevalence of blood disorders such as hemophilia A, hemophilia B, sickle cell disease, and thalassemia. These hematologic diseases, including Von Willebrand disease and rare anemia, are often characterized by coagulation factors like Factor VIII and Factor IX deficiencies, which lead to bleeding disorders. Gene therapy and enzyme replacement therapies are emerging treatments for these conditions, alongside blood transfusion and plasma therapy. In addition, innovations in anticoagulants, such as erythropoietin and iron chelation, offer new management options for patients with these rare hematological conditions. Advances in hematopoietic cells and bone marrow treatments are also making strides in improving patient outcomes.
Regulatory Approvals: The FDA's approval of gene therapies like Hemgenix and Beqvez has revolutionized treatment options for hemophilia B, offering potential cures with a single administration.
Strategic Portfolio Enhancements: Pharmaceutical companies are investing in expanding their portfolios to include treatments for rare hematologic diseases, driven by the potential for high returns and unmet medical needs.
Advancements in Diagnostic Technologies: The integration of genetic testing and molecular diagnostics has improved early detection and personalized treatment strategies.
High Treatment Costs: The introduction of gene therapies has led to unprecedented treatment costs. For instance, Hemgenix and Beqvez are priced at $3.5 million per dose, raising concerns about affordability and insurance coverage.
Manufacturing Complexities: The production of gene therapies involves complex processes, leading to challenges in scalability and consistency.
Regulatory Hurdles: Navigating the regulatory landscape for rare disease treatments can be time-consuming and costly, potentially delaying market entry.
See What’s Inside: Access a Free Sample of Our In-Depth Market Research Report.
Hemophilia: The hemophilia segment is experiencing significant growth, particularly with the advent of gene therapies that offer long-term solutions.
Sickle Cell Disease: Emerging gene therapies are showing promise in providing curative treatments, though challenges related to cost and accessibility remain.
Thalassemia: Ongoing research is focused on developing gene therapies to address this genetic blood disorder.
Myelodysplastic Syndromes: Treatment options are limited, with ongoing studies exploring potential therapeutic avenues.
Hospitals and Specialty Clinics: These institutions are primary centers for the administration of advanced therapies and management of rare hematologic conditions.
Research Institutes and Pharmaceutical Companies: Play a crucial role in the development of new treatments and conducting clinical trials.
North America: Dominates the market due to advanced healthcare infrastructure, high healthcare expenditure, and a favorable regulatory environment.
Europe and Asia: Show significant growth potential, driven by increasing awareness and healthcare improvements.
The rare hematology market is experiencing rapid development, driven by key players focused on innovative therapies and technologies. These companies are integral in advancing treatments for rare blood disorders, including hemophilia, sickle cell disease, and myeloma.
AbbVie Inc.
AstraZeneca Plc
Bayer AG
Biogen Inc.
bluebird bio Inc.
Bristol Myers Squibb Co.
CSL Ltd.
Emmaus Medical Inc.
F. Hoffmann La Roche Ltd.
GlaxoSmithKline Plc
Grifols SA
Jazz Pharmaceuticals Plc
Johnson & Johnson Services Inc.
Merck & Co. Inc.
Novartis AG
Novo Nordisk AS
Octapharma AG
Pfizer Inc.
Sanofi SA
Takeda Pharmaceutical Co. Ltd.
These companies are working through partnerships, acquisitions, and geographical expansions to enhance their positions in the market. Additionally, they are heavily investing in research and development to address unmet needs in rare hematology treatments.
Hematologic diseases like myelodysplasia, thrombocytopenia, and rare leukemias pose significant challenges for diagnosis and treatment. Genetic mutations and the need for specialized treatments such as hemoglobinopathy management, clotting factors like Factor XIII, and protein C, protein S, and antithrombin deficiencies are also critical components in patient care. The market is evolving with targeted therapies that address conditions like Gaucher disease, Fabry disease, and lysosomal storage disorders. The role of spleen disorders, hemorrhagic disorders, and hemolytic anemia in the treatment landscape is also significant, driving research into new treatment methods and approaches to improve the quality of life for affected individuals.
Investment in Gene Therapies: Companies should focus on developing cost-effective gene therapies to address the high treatment costs and improve patient access.
Regulatory Strategy: Engaging with regulatory bodies early in the development process can facilitate smoother approval pathways.
Collaborations and Partnerships: Forming alliances with research institutions and other pharmaceutical companies can enhance R&D capabilities and expedite time-to-market.
The rare hematology market is at a pivotal point, with technological advancements and regulatory support paving the way for innovative treatments. However, addressing the challenges of high treatment costs and manufacturing complexities will be crucial for sustainable growth.
Get more details by ordering the complete report
Affordability and Access: The high cost of gene therapies may limit patient access, particularly in low-resource settings.
Long-Term Efficacy and Safety: Ongoing monitoring and research are necessary to assess the long-term effects of gene therapies.
Market Competition: The entry of multiple companies into the gene therapy space may lead to increased competition and pricing pressures.
Policy Advocacy: Engage with policymakers to develop frameworks that support the affordability and accessibility of rare disease treatments.
Investment in Manufacturing Capabilities: Enhancing manufacturing processes can reduce costs and improve the scalability of gene therapies.
Patient-Centric Approaches: Develop treatment plans that consider the unique needs of patients, ensuring better outcomes and quality of life.
The rare hematology market presents significant opportunities for growth and innovation. By addressing the challenges of high treatment costs and focusing on patient-centric solutions, stakeholders can contribute to the advancement of care for individuals with rare blood disorders.
Safe and Secure SSL Encrypted