Pompe Disease Drugs Market Size 2024-2028
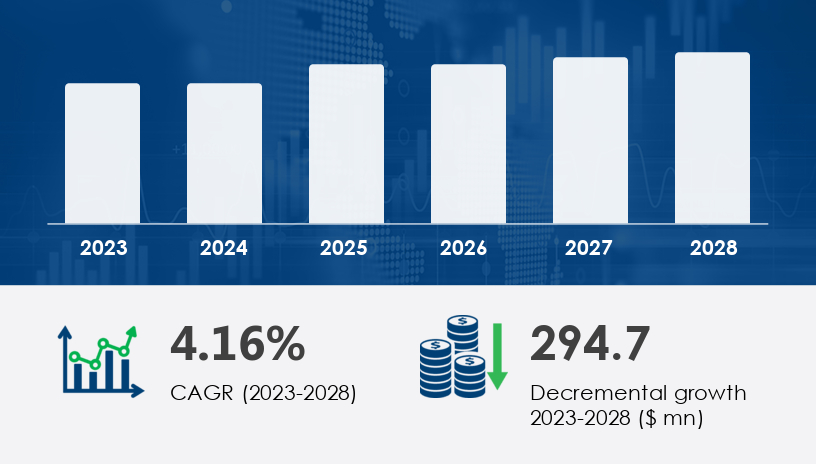
The Pompe disease drugs market is expected to grow by USD 294.7 million, at a CAGR of 4.16% from 2023 to 2028.
Pompe disease is a rare genetic disorder characterized by progressive muscle weakness and respiratory complications. The market growth is primarily driven by the increasing prevalence of the genetic mutation associated with the disease and the advancement of treatment options, such as Enzyme Replacement Therapy (ERT). Despite this, the high costs of treatment remain a significant challenge for both patients and healthcare systems.
For more details about the industry, get the PDF sample report for free
Pompe Disease Drugs Market Segmentation
The market is analyzed based on distribution channels, therapy types, and geographical regions.
Distribution Channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
Therapy
- Enzyme replacement therapy
- Substrate reduction therapy
- Others
Geography
- North America
- Europe
- Asia
- Rest of World (ROW)
By Distribution Channel Insights
The hospital pharmacies segment is expected to witness significant growth during the forecast period. These pharmacies are essential in facilitating the distribution and administration of medications to Pompe disease patients, particularly for those requiring specialized care.
Regional Market Trends
- North America: Estimated to contribute 41% to the global market growth during the forecast period.
- Europe: Germany and the UK are key players in the market within this region.
- Asia: Notable countries like China and Japan are emerging markets for Pompe disease treatments.
Market Dynamics
Drivers
- Increasing efforts to raise awareness about Pompe disease are a key driver of the market. As a rare genetic disorder, the disease often goes undiagnosed due to its rarity and diverse symptom manifestations. Efforts to educate healthcare professionals and the public are crucial for early detection and diagnosis.
Trends
- The approval of new drugs for Pompe disease is a prominent trend. A key development was the FDA's approval of Amicus Therapeutics' dual-component therapy, Pombiliti (cipaglucosidase alfa-atga) + Opfolda (miglustat) 65mg capsules, in September 2023. This therapy, aimed at adults with late-onset Pompe disease, marks an important advancement in treating the disease, especially for patients who have not responded to existing enzyme replacement therapies.
Challenges
- The high treatment costs associated with Pompe disease remain a significant challenge. The disease's rarity and the complexity of treatment make it expensive, putting a strain on healthcare systems and insurance providers. However, ongoing research may lead to more affordable treatment options in the future.
Get more details by ordering the complete report
Key Players in the Pompe Disease Drugs Market
The following companies are key players in the Pompe disease drugs market:
- Amicus Therapeutics Inc.
- Astellas Pharma Inc.
- AVROBIO Inc.
- Bayer AG
- EpiVax Inc.
- F. Hoffmann La Roche Ltd.
- JCR Pharmaceticals Co. Ltd.
- Johnson and Johnson
- Maze Therapeutics Inc.
- Sanofi SA
These companies are engaging in strategic initiatives such as partnerships, product launches, and geographical expansions to strengthen their presence in the Pompe disease market.
Recent Developments
- September 2023: The FDA approved Amicus Therapeutics' dual-component therapy, Pombiliti + Opfolda capsules, for late-onset Pompe disease patients.
Pompe disease is a rare genetic disorder caused by the deficiency of the enzyme acid alpha-glucosidase. Medical advancements are pivotal in the ongoing search for effective treatments. The growing understanding of the disease and improved drug options, particularly enzyme replacement therapy and gene therapy, offer hope to affected individuals. The regulatory framework and healthcare infrastructure are critical in ensuring that these advancements are accessible to patients worldwide.