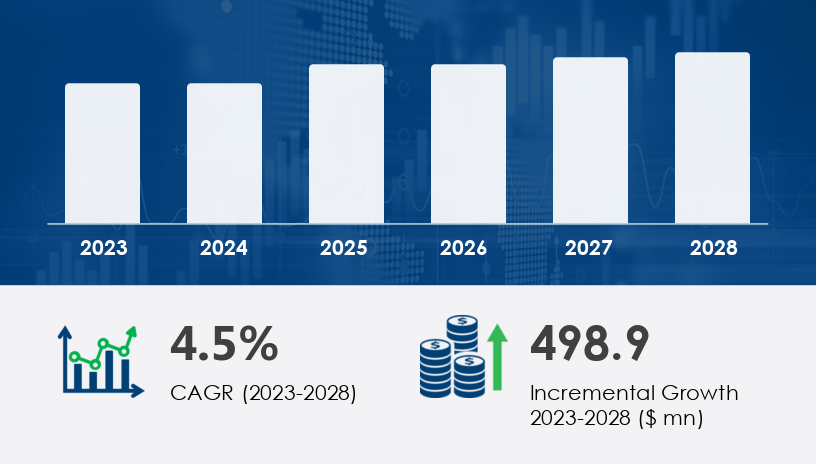
The France neonatal and prenatal devices market is projected to grow by USD 498.9 million at a CAGR of 4.5% between 2023 and 2028. This growth is primarily driven by the increasing incidence of preterm births and a rising awareness of fetal screening and monitoring procedures. However, affordability issues remain a significant challenge for many healthcare providers and patients, limiting access to advanced medical technologies in neonatal and prenatal care.
For more details about the industry, get the PDF sample report for free
The hospitals segment is expected to see significant growth throughout the forecast period. Hospitals, particularly those with neonatal intensive care units (NICUs), remain the primary end-users of neonatal and prenatal medical technologies. Specialized clinical teams in these hospitals focus on managing high-risk infants, extremely preterm babies, and birth-related complications.
Key devices used in hospital NICUs include:
Innovative neonatal therapies and diagnostics continue to emerge, improving health outcomes for premature and high-risk infants. Technologies such as delayed cord clamping, LifeStart, Inspire, and Concord are being integrated into hospital practices to enhance newborn care.
Hospitals accounted for a market value of USD 983.60 million in 2018 and have demonstrated a steady increase throughout the forecast period.
Preterm birth, defined as the delivery of a baby before 37 weeks of gestation, remains a critical health concern in France. Several risk factors contribute to preterm births, including:
Preterm births are categorized into three groups based on gestational age:
Premature babies face a higher risk of mortality and long-term health complications, including cerebral palsy, learning disabilities, sensory impairments, developmental delays, and respiratory issues. Neonatal and prenatal devices play a crucial role in monitoring and supporting premature infants, improving overall health outcomes and reducing neonatal mortality rates.
The France neonatal and prenatal devices market is witnessing an increase in demand for fetal screening and monitoring technologies due to rising concerns about neonatal mortality and genetic disorders. Genetic disorders, including Down syndrome and other chromosomal abnormalities, contribute significantly to stillbirths and infant mortality.
Several French authorities and private organizations are actively promoting prenatal screening initiatives. Expecting mothers are increasingly adopting non-invasive prenatal testing (NIPT) and other screening methods to detect potential genetic defects early. This growing awareness and adoption of advanced screening methods are fueling the market expansion.
Despite technological advancements, high costs remain a major barrier in the France neonatal and prenatal devices market. The cost of neonatal intensive care unit (NICU) treatments exceeds USD 125 per patient per day, making access to these critical healthcare services difficult for many middle-class families.
The financial burden on healthcare institutions and families limits the widespread adoption of neonatal and prenatal devices in hospitals and diagnostic centers. The high initial investment and maintenance costs of these devices further impede market growth.
The neonatal and prenatal devices market is experiencing significant growth, driven by the increasing demand for fetal monitoring systems and neonatal incubators in healthcare facilities. Technological advancements in phototherapy equipment, infant warmers, and neonatal ventilators have enhanced the ability of healthcare providers to manage preterm births effectively. The rising adoption of prenatal ultrasound devices and fetal Doppler monitors supports early detection of fetal health complications, contributing to improved maternal and infant care. Additionally, neonatal resuscitation units and pulse oximetry for neonates are becoming essential in premature infant care, ensuring real-time monitoring of critical health parameters. As the focus on maternal health monitoring intensifies, the demand for NICU equipment continues to expand, reinforcing the need for specialized medical technologies in neonatal and prenatal care.
Get more details by ordering the complete report
The France neonatal and prenatal devices market includes several prominent companies engaged in product innovation, mergers and acquisitions, strategic alliances, and geographical expansion. The major key players operating in the market are:
Innovations in non-invasive prenatal testing and neonatal apnea monitors are transforming the landscape of portable prenatal devices, providing greater accessibility and accuracy in advanced neonatal care. The development of prenatal diagnostics technology has led to improved screening techniques, aiding in the early detection of genetic abnormalities and fetal health risks. The integration of neonatal and prenatal market trends into healthcare strategies reflects the growing emphasis on enhancing neonatal survival rates and maternal well-being. The introduction of cutting-edge solutions in prenatal diagnostics technology ensures better outcomes for newborns and expectant mothers. With continuous advancements in neonatal and prenatal care, medical institutions are increasingly investing in state-of-the-art equipment to meet the evolving needs of healthcare professionals and patients.
Safe and Secure SSL Encrypted