Breast Cancer Liquid Biopsy Testing Devices Market 2024-2028: Size and High costs associated with liquid biopsy testing
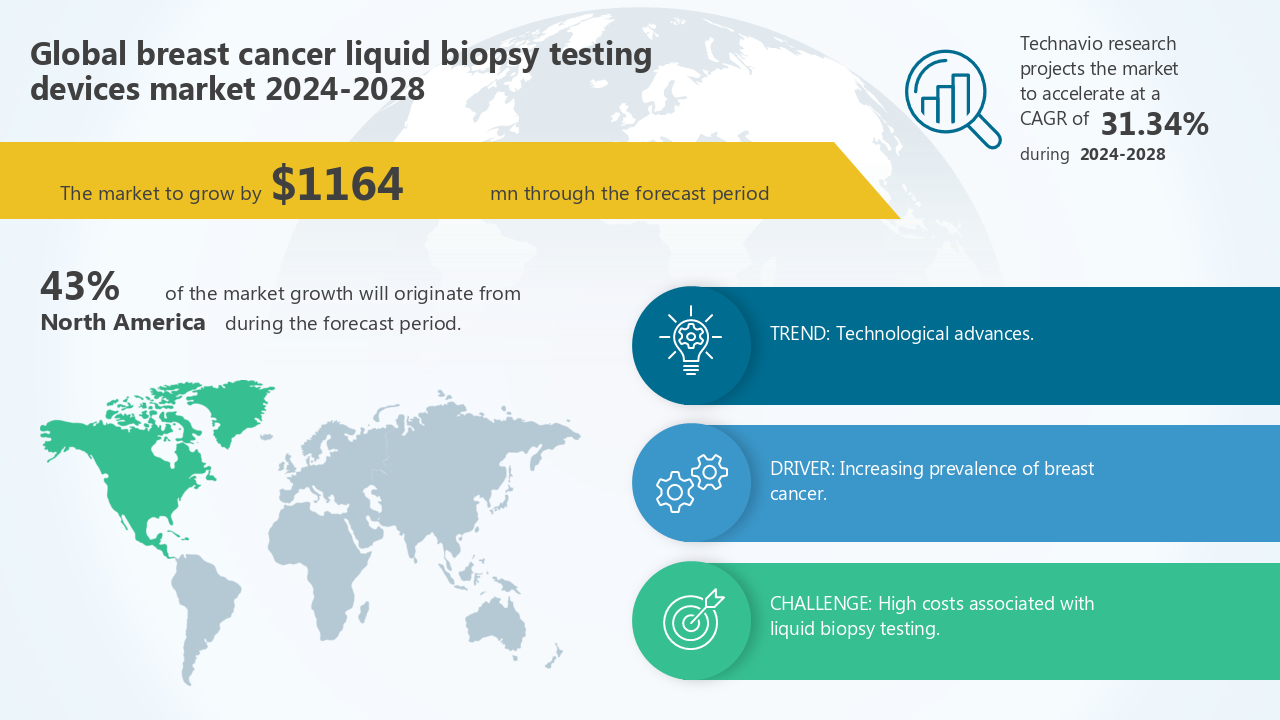
The breast cancer liquid biopsy testing devices market size by Type (CTCs and circulating nucleic acids and Extracellular vesicles) and Geography (North America, Europe, Asia, and Rest of World (ROW)), is forecast to increase by USD 1164 million. The market is expected to grow at a CAGR of 31.34% between 2024 and 2028.
- In the realm of breast cancer diagnostics, liquid biopsy testing devices have emerged as a preferred alternative to traditional methods. These non-invasive procedures offer healthcare professionals deeper insights into the tumor's molecular makeup by analyzing circulating tumor cells and biomarkers in the blood. The increasing demand for early and accurate breast cancer detection, coupled with the growing number of individuals suspected of having the disease, has fueled the global adoption of these advanced diagnostic solutions. By employing sensitive and specific molecular diagnostic techniques, liquid biopsy enables comprehensive cancer profiling, providing valuable information for personalized treatment plans.
- The Breast Cancer Liquid Biopsy Testing Devices market represents a significant growth opportunity for businesses in the diagnostics industry. These devices enable the non-invasive detection of breast cancer through the analysis of circulating tumor cells and biomarkers in bodily fluids. Market expansion is driven by rising incidences of breast cancer, increasing demand for minimally invasive diagnostic procedures, and technological advancements in liquid biopsy testing. Companies investing in this sector can capitalize on these trends and contribute to improving patient outcomes and advancing cancer research.
Access the full report to know who are the other key countries segment-wise forecast and historic data
Some of the Key Companies:
- A. Menarini Industrie Farmaceutiche Riunite Srl
- Bio Rad Laboratories Inc.
- Cell Microsystems Inc.
- Exact Sciences Corp.
- F. Hoffmann La Roche Ltd.
- Guardant Health Inc.
- Illumina Inc.
- Isogen Life Science BV
- Mesa Laboratories Inc.
- Myriad Genetics Inc.
- Natera Inc.
- NeoGenomics Laboratories Inc.
- Novogene Co. Ltd.
- OncoDNA
- Pfizer Inc.
- QIAGEN NV
- SAGA Diagnostics AB
- Sysmex Corp.
- Thermo Fisher Scientific Inc.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: media@technavio.com
Website: www.technavio.com/