Cell and Gene Therapy Market Size 2024-2028
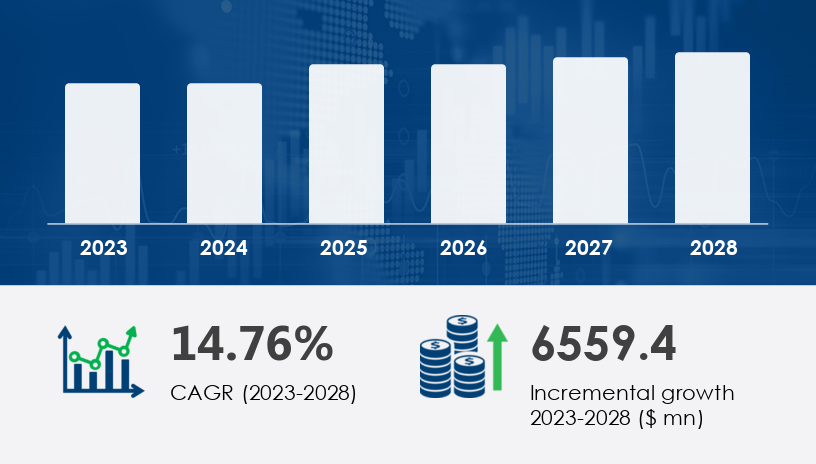
The cell and gene therapy market is forecast to increase by USD 6.56 billion at a CAGR of 14.76% between 2023 and 2028. This growth is primarily driven by the rising prevalence of chronic diseases, which boosts the demand for innovative treatments, including both cell replacement and gene augmentation therapies. Strategic alliances between industry players and academic institutions are pushing advancements in biomanufacturing and single-use technology, which streamline production processes and reduce costs. However, the high costs of development and the failure rate in clinical trials pose ongoing challenges, requiring continued investment in research and development to ensure the industry’s success. Apheresis technology, which allows for the selective removal of cells from the bloodstream, is critical in cell and gene therapy, enabling the collection and processing of therapeutic cells.
For more details about the industry, get the PDF sample report for free
Market Segmentation
The market is analyzed across various segments and regions, with forecasts provided for the period 2024-2028 and historical data from 2018-2022.
By Type:
- Cell Therapy: Expected to witness significant growth due to its potential for personalized medicine, utilizing both autologous and allogeneic cells. Cell therapy is especially promising for chronic disease treatments, particularly in hematology, ophthalmology, and neurological disorders.
- Gene Therapy: Focuses on using genetic material to correct or prevent diseases, including innovative approaches such as gene silencing, gene augmentation, and CRISPR technology.
By Geography:
- North America
- Europe
- Asia
- Rest of World (ROW)
Market Dynamics
Drivers:
- Rising Prevalence of Chronic Diseases: As chronic diseases become more common, there is an increasing unmet medical need for advanced therapies like gene and cell therapies.
- Advancements in Biotechnology: Technologies such as CRISPR-Cas9 gene editing are opening up new opportunities for therapeutic interventions.
- Increased Investment: Public and private sector investments are essential for accelerating the development of cell and gene therapies.
Trends:
- Personalized Medicine: A growing trend in cell and gene therapy is tailoring treatments to individual patients’ genetic profiles, enhancing treatment efficacy.
- Combination Therapies: There is a trend toward combining cell and gene therapies with other treatment modalities, such as immunotherapy, for improved outcomes.
- Regulatory Landscape: Clear and efficient regulatory frameworks are vital for speeding up the development and commercialization of cell and gene therapies.
Challenges:
- High Development Costs: The process of developing cell and gene therapies is expensive and requires substantial investment.
- Manufacturing Challenges: Scaling up the production of cell and gene therapies to meet market demand remains a key challenge.
- Regulatory Hurdles: Navigating the regulatory process for new therapies can be time-consuming and costly.
Get more details by ordering the complete report
Key Companies and Market Insights
Strategic alliances, mergers, acquisitions, geographical expansions, and new product launches are some of the strategies companies are adopting to enhance their presence in the market. Key players in the cell and gene therapy market include:
- Alnylam Pharmaceuticals Inc.
- Amgen Inc.
- bluebird bio Inc.
- Bristol Myers Squibb Co.
- Castle Creek Biosciences Inc.
- CORESTEM Inc.
- Cytori Therapeutics Inc.
- Dendreon Pharmaceuticals LLC
- Ferring BV
- Gilead Sciences Inc.
- Helixmith Co. Ltd.
- Human Stem Cells Institute
- JCR Pharmaceticals Co. Ltd.
- Kolon TissueGene Inc.
- Novartis AG
- Orchard Therapeutics Plc
- Pfizer Inc.
- Sibiono GeneTech Co. Ltd.
- Vericel Corp.
Recent Developments
- Biogen Inc. launched cotoretigene toliparvovec, a gene therapy for treating X-linked retinitis pigmentosa.