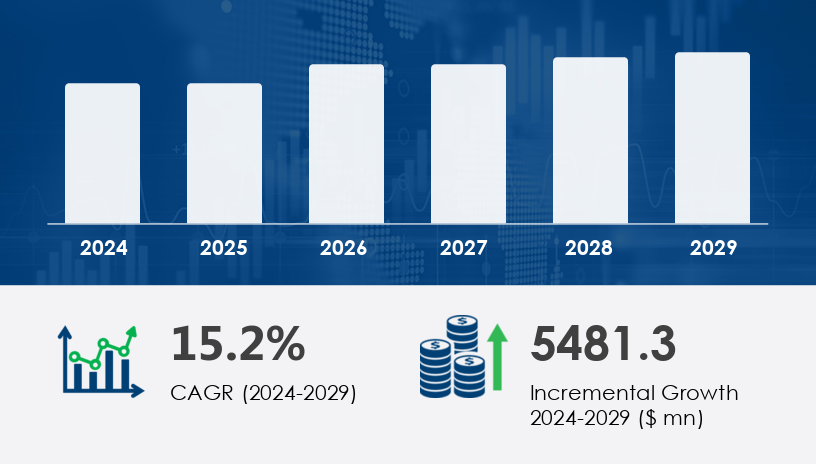
The global biopharmaceutical analytical testing services market is poised for significant expansion, with an anticipated increase of USD 5.48 billion from 2024 to 2029, reflecting a robust compound annual growth rate (CAGR) of 15.2%. This growth is propelled by escalating demand for monoclonal antibodies (mAbs), biosimilars, and the imperative for stringent regulatory compliance in drug development processes. However, challenges such as the scarcity of skilled professionals and the complexities of analytical method validation pose potential obstacles to this upward trajectory.
For more details about the industry, get the PDF sample report for free
The Biopharmaceutical Analytical Testing Services Market is expanding rapidly due to the increasing complexity of modern therapeutics such as monoclonal antibodies, biosimilars, and therapeutic proteins. These biologics require in-depth protein characterization, along with rigorous impurity profiling and stability testing to ensure drug purity and biological potency throughout the product lifecycle. Services including method validation, immunogenicity testing, pharmacokinetics, and assessments of bioavailability and bioequivalence are critical in advancing safe and effective treatments. The market also supports toxicology studies, drug release analysis, and metabolite analysis in various sample types such as biological fluids and tissue extracts, ensuring robust quality control aligned with global regulatory compliance standards.
Monoclonal antibodies, engineered to target specific antigens, have become pivotal in treating various conditions, including cancer, autoimmune disorders, and infectious diseases. The increasing prevalence of these diseases has escalated the need for mAb-based therapies, thereby driving the demand for comprehensive analytical testing services to ensure the safety and efficacy of these biologics.
The integration of cutting-edge analytical techniques, such as mass spectrometry, chromatography, and immunoassays, has enhanced the precision and efficiency of bioanalytical testing. These advancements facilitate the accurate characterization of complex biologics, ensuring compliance with stringent regulatory standards and expediting the drug development process.
Adherence to international guidelines, including those set by the International Council for Harmonization (ICH), is paramount in the development of biopharmaceuticals. Analytical testing services play a crucial role in validating analytical methods, ensuring product quality, and meeting regulatory requirements, thereby mitigating risks associated with non-compliance.
The complexity of biopharmaceuticals necessitates a highly skilled workforce proficient in advanced analytical techniques. The current shortage of trained professionals in the field poses a significant challenge, potentially hindering the capacity to meet the growing demand for analytical testing services.
The validation of analytical methods is a critical component in ensuring the reliability and reproducibility of test results. However, the intricate nature of these methods, coupled with evolving regulatory requirements, presents ongoing challenges for service providers in maintaining validated processes.
See What’s Inside: Access a Free Sample of Our In-Depth Market Research Report.
Bioanalytical Testing: This segment is projected to witness significant growth, driven by the increasing need for pharmacokinetic and toxicological assessments in drug development.
Method Development and Validation: Essential for establishing reliable and reproducible testing protocols, this service ensures compliance with regulatory standards.
Stability Testing: Critical for determining the shelf-life and storage conditions of biopharmaceuticals, stability testing ensures product integrity over time.
Others: Includes specialized testing services tailored to specific client needs, contributing to the overall market expansion.
Pharmaceutical and Biotech Companies: These entities are the primary consumers of analytical testing services, relying on them to ensure the quality and safety of their products.
Contract Research Organizations (CROs): CROs provide outsourced research services, including analytical testing, to pharmaceutical and biotechnology companies, facilitating efficient drug development processes.
North America: Dominates the market, accounting for a significant share due to the presence of leading pharmaceutical companies and advanced healthcare infrastructure.
Europe: Experiences steady growth, driven by stringent regulatory frameworks and a strong emphasis on research and development.
Asia-Pacific: Emerging as a rapidly growing market, attributed to increasing investments in healthcare and biotechnology sectors, particularly in countries like China and India.
South America and MEA
Investment in Workforce Development: Addressing the shortage of skilled professionals through targeted training programs and educational initiatives is crucial to sustaining market growth.
Adoption of Advanced Analytical Technologies: Embracing innovations in analytical methodologies can enhance testing efficiency and accuracy, meeting the evolving demands of the biopharmaceutical industry.
Strengthening Regulatory Compliance Frameworks: Continuous alignment with international regulatory standards ensures the reliability and acceptance of analytical testing services across global markets.
In-depth research analysis highlights the pivotal role of GMP testing and adherence to ICH guidelines in supporting efficient drug development across a broad spectrum of applications, from biologics testing to personalized medicine. Analytical services are essential for evaluating drugs for oncology, infectious diseases, and chronic diseases, requiring advanced techniques like mass spectrometry, chromatography, and a range of bioanalytical assays. The growing adoption of ELISA testing, multiplex assays, and biomarker analysis reflects the demand for precise and sensitive diagnostics. To maintain data integrity, the testing of raw materials, drug substances, and products in clinical trials includes meticulous sample preparation and ensures data reliability—a cornerstone in the advancement of safe and effective biopharmaceuticals.
The biopharmaceutical analytical testing services market is poised for continued expansion, driven by technological advancements and increasing demand for biologic therapies. Service providers must navigate challenges related to workforce shortages and method validation complexities to capitalize on emerging opportunities. Strategic investments in innovation and compliance will be pivotal in shaping the future landscape of the industry.
Regulatory Hurdles: Navigating the complex and evolving regulatory environment requires agility and proactive compliance strategies.
Technological Integration: Seamlessly incorporating advanced analytical technologies necessitates substantial investment and expertise.
Market Competition: Intensifying competition among service providers underscores the need for differentiation through quality, innovation, and customer service.
Get more details by ordering the complete report
Enhance Training Programs: Develop and implement comprehensive training initiatives to cultivate a skilled workforce adept in advanced analytical techniques.
Invest in Research and Development: Allocate resources towards the development of innovative testing methodologies to stay ahead of industry trends.
Foster Strategic Partnerships: Collaborate with academic institutions, research organizations, and industry leaders to drive innovation and expand service offerings.
The biopharmaceutical analytical testing services market is at a pivotal juncture, characterized by rapid growth and evolving challenges. Stakeholders must adopt a proactive approach, focusing on workforce development, technological innovation, and regulatory compliance to navigate the complexities of the industry successfully.
Safe and Secure SSL Encrypted